By Aurelia, Dieke and Muhammed
The ongoing COVID-19 pandemic has left the European Union (EU) to deal with severe medicinal challenges. Rising infection numbers have led to a race against time to find a treatment. This blogpost delves into the role of the European Medicines Agency (EMA). To fulfill the need of more promptly developed medicine within the current health crisis, the EMA started fast-track procedures for treatments and vaccines for COVID-19. The agency is therefore proving to be as important as never before. To effectively reply to the negative public perceptions of the EU’s COVID crisis management, the EMA should play an even more important and autonomous role and needs to be included in the future health policy plans of the EU.
How does a medicinal product access the EU market?
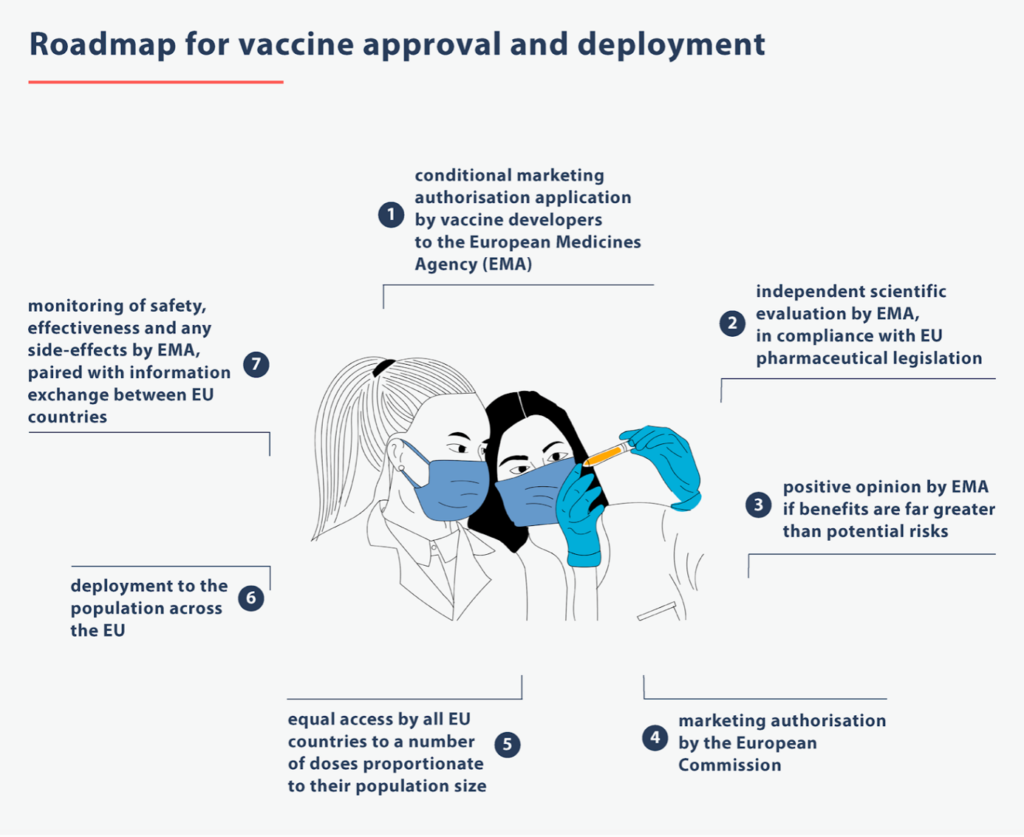
Together with the European Commission, the EMA is responsible for authorizing medicines on the EU market. Before a medicinal product can access the European Market, it first has to be assessed and reviewed by the EMA. Companies have to prove that their product is safe to use for patients by providing the EMA with the necessary documents regarding the effect and consequences of the medicines. After thorough assessment, the EMA gives a decision to the Commission who grants authorization for distribution of the medicine. In this so-called centralised procedure, companies can let their product access the market on several or all EU Member States at once, without having to apply to each single Member State (decentralised procedure). After the product is distributed on the market, EMA will also monitor its effects to ensure patients’ safety throughout the EU.
Additional tools of the EMA to fight against COVID-19
The COVID-19 pandemic required the EMA to take on tasks that required ‘ad hoc’ working methods. The role of the EMA during the pandemic has contributed to global efforts to save lives. It has accelerated the development and the approval procedure for safe and effective treatments and medicines, specifically in case of vaccine against COVID-19. The EMA also has contributed to ensuring the continuation of assessment and monitoring of the medicines. It was thereby also ensuring that patients and healthcare professions in the EU have access to medicines for all needs during the pandemic as well as access to reliable information.
The EMA has established different task forces and groups that are dedicated to deal with scientific, regulatory and operational challenges that have come to light by the COVID-19 pandemic. The COVID-19 EMA Pandemic Task Force has been established to help both the Member States and the European Commission in taking rapid and coordinated regulatory action regarding the development, authorization and (safety) monitoring of treatments and vaccines for COVID-19. Moreover, an EMA COVID-19 Steering Group has been established in order to respond rapidly to the evolving scientific regulatory challenges and to safeguard the main activities of the EMA.
Most remarkable in the newly introduced fast-track procedures are the so-called „rolling reviews“. Instead of going through the ordinary centralised authorization procedure and having to wait until the complete information on research is submitted, the EMA now assesses data for promising medicines or vaccines as they become available, even though the developing process is not yet finished. This allows for the EMA to limit the duration of the review procedure immensely from formerly 210 days per review cycle to now only around two weeks.
EMA’s role post COVID-19 – evolving?
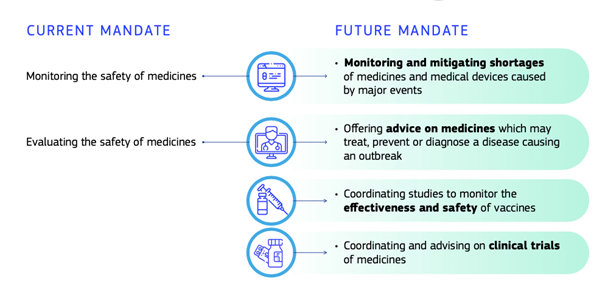
The COVID-19 pandemic has led to additional ‘burdens’ for the work programme of the EMA, next to its core tasks. The EMA has already informed the European Commission that it needs additional staff to respond to the pandemic as well as a framework in place to tackle public health emergencies where additional expertise is provided for.
Furthermore, political debates about the competences of the EU in the public health sector have been triggered. Growing expectations by EU citizens towards the EU to face the current medicinal challenges clash with the limited competences the EU has in this sector, as public health regulation lays mainly in the hands of the Member States (see Art. 168 (2) TFEU). The European Commission and the European Parliament have therefore stressed for the creation of a European Health Union. This also foresees a legislative proposal to enforce the mandate of the EMA. The proposal aims at improving crisis preparedness and the management and monitoring of medicinal products and medical devices. It introduces a legal framework in which new task forces and steering groups are established within the agency to prevent shortages of medicinal products and devices etc. With the creation of a European Health Union as seen from the proposal, the EMA is provided with more tasks. Therefore, the European Commission expressed that in order for the EMA to carry out future tasks additional to their current mandate, it requires additional staff.
Could the EMA get more tasks in the future?
EMA enjoys an advisory role for the approval and monitoring of the medicines. But since these are very technical issues, the Commission does not have the scientific expertise to oppose the recommendations of EMA and thus simply adopts them. This makes the EMA a quasi-regulatory agency, that does not only give a recommendation but also has the de facto power to enforce it. Nevertheless, that means that the recommendation of the EMA cannot be judicially reviewed in a proper way, because ultimately, the EMA is not the one responsible for the coming into force of it. In the establishment of the EMA, the Meroni-Romano doctrine was effective in delegating powers. According to that doctrine, the possibility by EU institutions to delegate powers is very limited and subject to conditions that are difficult to fulfill.
However, the recent ESMA Short Selling case allows for a more general delegation of powers to the agencies. If EMA can have more powers under the ESMA Short-Selling doctrine, it would be the realization of the current de facto enforcement of the EMA recommendations. This could switch the current control-mechanism from inefficient ex ante control of the EMA decisions by the Commission to the ex post accountability EMA’s decisions before EU Courts. Therefore, the role of the EMA can be strengthened in addressing a health crisis, as the COVID-19 pandemic showed us the importance of the EMA’s role and the aspiration for building a European Health Union. However, this also raises questions regarding the limited competence of the EU in the field of public health, and a sensitive area for the Member States to regulate.
